New Developments in Male Contraception
Authors
INTRODUCTION
The world's population, which currently exceeds 6 billion, is increasing by 80 million per year.1 Throughout the world, population growth is producing significant problems with food and water supply, pollution, overcrowding, and resource depletion. Much of this population growth is unintended. Family planning organizations estimate that half of all conceptions are unplanned and half the resulting pregnancies are undesired.2 In part, this high rate of unintended pregnancy is due to unavailable or inadequate contraception. Undesired pregnancies often end in abortion or infanticide. In the United States, where contraception is widely available, there are 1.5 million abortions yearly, accounting for roughly one quarter of all pregnancies.3 Abortion in countries where the procedure is illegal or unavailable from trained providers with sterile equipment can be lethal and is estimated to kill between 50,000 and 100,000 women yearly worldwide.4, 5 Although current contraceptives for women can be extremely effective at preventing pregnancy, many women either do not have access to them or choose not to use them because they find the side-effects unacceptable or perceive the health risks to be too great. Thus, a pressing need remains for additional contraceptive options, especially male-directed varieties.
At present, the few methods of contraception available to men include condoms, withdrawal, spermicides, and vasectomy; and vasectomy is not reliably reversible. Efforts are under way to develop newer male contraceptives to allow men to become full partners in the prevention of unintended pregnancy. This chapter first reviews existing methods of male contraception and then discusses the current status of research into the development of novel approaches to male fertility control
MALE REPRODUCTIVE PHYSIOLOGY
The testes are responsible for both the production of sperm and the synthesis of testosterone, a steroid hormone. Testosterone is important in sperm production and also in the maintenance of male libido, muscle, and bone mass. In addition, testosterone has a beneficial impact on mood, cognition, and overall sense of well-being.6 Testosterone is produced by Leydig cells within the interstitium of the testes, and its production is stimulated by luteinizing hormone (LH). Sperm are produced within the seminiferous tubules, where their maturation is nurtured by Sertoli cells under the influence of follicle-stimulating hormone (FSH) and high levels of intratesticular testosterone.
Production of mature sperm from germ cells takes approximately 72 days. Sperm production by the testis is continuous and occurs in four distinct phases: (1) a mitotic phase in which the germ cells, the spermatogonia, give rise to diploid spermatocytes; (2) a meiotic phase in which spermatocytes double their chromosome complement and undergo two cycles of cell division resulting in haploid spermatids; (3) spermiogenesis, which involves spermatid nuclear condensation and flagellum formation; and (4) spermiation, which involves release of the spermatozoa into the tubular lumen.7 Storage and further maturation of sperm take place in the epididymis; sperm aspirated from the epididymis are capable of fertilization.8
As in most endocrine systems, an elaborate feedback loop exists to regulate hormone production and testicular function. Production of pituitary gonadotrophins FSH and LH in the anterior pituitary is regulated by gonadotropin-releasing hormone (GnRH), a decapeptide secreted by the hypothalamus in a pulsatile fashion (Fig. 1). Testosterone and inhibin B, a glycoprotein hormone secreted by Sertoli cells, provide negative feedback on the production of GnRH and pituitary gonadotropins at the hypothalamus (testosterone) and pituitary (FSH and testosterone). Given the nature of sperm production, a male contraceptive can work in one of three ways: (1) by preventing sperm from reaching the egg by physical barriers (condoms and vasectomy), (2) by preventing sperm production (hormonal methods), or (3) by killing or inhibiting the function of sperm (spermicides and sperm vaccines).
BARRIERS TO SPERM
Vasectomy
Vasectomy is a safe, simple, outpatient surgery performed under local anesthesia in which the ductus deferens is severed and the ends ligated through a small scrotal incision. There are approximately 500,000 vasectomies performed in the United States yearly and worldwide over 50 million men have undergone the procedure.9 Vasectomies are highly effective with a failure rate of less than 1% and a low incidence of complications.10, 11 The relatively new “no-scapel technique,” which was perfected in Sichuan province in China,12 in which a single puncture is made midline in the scrotal raphe with scissors, is probably superior to older techniques.13, 14 Drawbacks to vasectomy include a delay in the onset of azoospermia of 3–6 months, pain, and rare infections of the surgical site. Although most postoperative pain resolves quickly, upward of one third of men do experience chronic testicular discomfort.15 In one study of such men, 27 of 33 had relief of these symptoms with reversal of the vasectomy.16
Vasectomies are most appropriate for men who no longer wish to father children. However, 3–5% of men with vasectomies do eventually request reversal, usually due to remarriage.17 Vasectomy reversal, a procedure termed vasovasostomy, has the potential to restore fertility, however, rates of pregnancy vary from 30 to 70% depending on the length of time between the vasectomy and the reversal procedure. In 20–30% of men, vasovasostomy is unable to restore patency of the vas if more than 8 years have elapsed since the original vasectomy.18 In addition, 20–40% of men remain infertile despite restored patency of the vas (as documented by imaging techniques) possibly due to the presence of antisperm antibodies.19 For these reasons, vasectomy cannot be recommended as a truly reversible method of contraception.
The good news about vasectomy is that it appears to be safe in terms of overall male health. Earlier reports of associations between vasectomy and cardiovascular disease have proven incorrect, and more recent concerns about vasectomy and prostate cancer risk have not been substantiated.20 In summary, vasectomy is highly effective and very safe. The major drawbacks are chronic testicular discomfort in about one third of men and the inability of surgery to reliably restore fertility when desired.
Condoms
Condoms made of animal intestine have been used as a means of male fertility control for several hundred years. Since 1920, most condoms have been made of latex rubber, which offers some protection against many sexually transmitted diseases including human immunodeficiency virus. Condoms are mostly free from adverse side-effects; however, condoms have a marginal contraceptive efficacy, which results for the most part from improper or inconsistent usage, although condom breakage occurs in up to 2% of cases. Pregnancy rates for couples using condoms as their sole means of contraception approach 15–20% per year.21 A drawback to condoms is their poor long-term compliance because they must be used correctly during 100% of sexual encounters. In addition, some men dislike condoms because they feel that condoms either diminish sexual pleasure or are difficult to use. Finally, some men and women develop allergic reactions to the latex (derived from rubber plants) that can cause skin irritation and, rarely, anaphylaxis.22, 23
HORMONAL MALE CONTRACEPTION
Because of the drawbacks of existing methods of male contraception, efforts have been made to develop a hormonally derived contraceptive analogous to estrogen/progesterone birth control pill for women. Such a hormonal contraceptive has the potential to be safe, easy to use, and reversible. Testosterone, when administered in slightly supraphysiologic doses, can function as a contraceptive by suppressing the secretion of the pituitary gonadotropins LH and FSH. Low levels of LH and FSH deprive the testis of the signals required for spermatogenesis, leading to markedly decreased sperm counts in most, but not all, men. Sperm counts uniformly rise after the cessation of testosterone administration. Surveys conducted in several countries suggest that such a hormonally derived male contraceptive administered by either daily pills or periodic injections would be welcomed by a large percentage of men and women.24, 25
In general, hormonal contraceptives do not incapacitate existing sperm; they block the initiation of sperm production. Given that sperm take an average of 72 days to reach maturity, it is likely that any contraceptive based on manipulation of the hormonal axis must be associated with some delay in the onset of full contraceptive effect. In normal men, sperm counts vary from 20–200 million sperm per milliliter of ejaculate. The absence of spermatozoa in the ejaculate, a condition termed azoospermia, renders fertilization impossible and is therefore the ultimate goal of hormonal male contraceptives. Most studies to date, however, demonstrate that some men sustain partial but incomplete reduction of their sperm counts, a condition called oligozoospermia.26 Sperm counts below 3 million sperm per milliliter of ejaculate are associated with decreased rates of pregnancy.27 Severe oligozoospermia (counts less than 1 million sperm per milliliter) decreases the chances of conception even further and is therefore considered a reasonable short-term goal for male contraceptive research.
Ethnic differences must be considered in interpreting results of contraceptive trials. Study volunteers in Asia are more susceptible to testosterone-induced suppression of spermatogenesis, with rates of azoospermia in the 90–100% range. Men studied in Europe, North America, and Australia, however, have rates of azoospermia closer to 60–80% on the same regimens.27, 28 Although the explanation for this difference is inconclusive, it is important in the interpretation of trial results and complicates extrapolation of data to different populations.
Testosterone as contraceptive
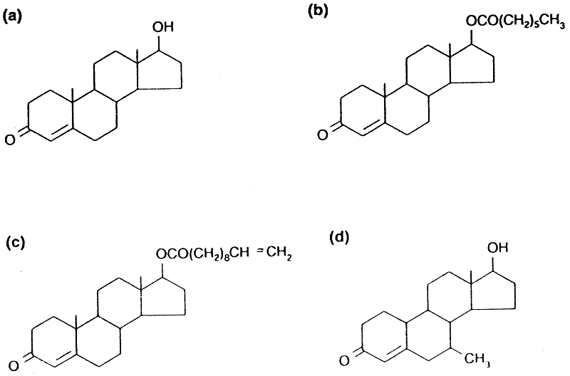
Administration of unmodified testosterone (Fig. 2A) is impractical because when given orally or by injection it is quickly degraded by the liver. Therefore, most hormonal contraceptive regimens have used longer-acting injectable testosterone esters such as testosterone enanthate (TE) (see Fig. 2B) given by intramuscular injection on a weekly to fortnightly basis. On such a regimen, sperm counts approach 0 between 2 and 3 months, with recovery of normal sperm counts 3–4 months after the injections are discontinued.
Two multicenter trials of TE as a male contraceptive have been conducted by the World Health Organization (WHO). The first study enrolled 271 subjects who were given 200 mg TE intramuscularly weekly.28 Of these men, 60% achieved azoospermia, and an additional 30% were rendered severely oligozoospermic. Moreover, 119 of the men who became azoospermic were instructed to discontinue other birth control and to continue on TE injections. These couples were followed up for 1 year. During that time, only 1 pregnancy occurred, thereby demonstrating testosterone-induced azoospermia to be an effective contraceptive.
The second WHO study examined the fertility of both the men who became azoospermic and the men who achieved severe oligozoospermia with TE injections.27 In total, 399 men were enrolled in this study. Of these, 98% became severely oligospermic (less than 3 million sperm per milliliter) or azoospermic. There were no pregnancies caused by the men who became azoospermic, and in men who became severely oligospermic, fertility was reduced to 8.1 pregnancies per 100 person-years. The overall failure rate (including men who failed to suppress their sperm counts) was 3.4%, for an overall contraceptive efficacy of 96.6%. In both groups, sperm counts returned to normal after the cessation of testosterone injections, and there were no major side-effects.
These studies demonstrated that injected TE is safe, fully reversible, and effective as a contraceptive in most men; however, a proportion of men fail to suppress below 3 million sperm per milliliter and therefore presumably remain fertile. In addition, the necessity of weekly intramuscular injections is a deterrent. Twelve per cent of patients in the second WHO study discontinued involvement for personal or medical reasons, or due to dislike of the injection schedule. Finally, high-dose TE has been shown to decrease serum HDL cholesterol, which could accelerate atherosclerosis.29, 30
Because of poor acceptability of weekly injections, newer methods of sustained testosterone delivery suitable for use in a contraceptive regimen are being pursued. Testosterone undecanoate (TU) (see Fig. 2C), is a long-chain ester that results in normal serum T levels for at least 6 weeks in hypogonadal men.31, 32, 33
Two large trials of TU injections have been conducted in China.34 Volunteers received monthly injections of 500 or 1000 mg TU. Almost 90% of men suppressed spermatogenesis below 1 million sperm/ml and were allowed to use the injection as their sole method of contraception. Very few pregnancies were reported in treated men and side-effects were minimal. Unfortunately, this approach to contraception was not approved for use in China.
The testosterone ester 7α-methyl-19-nortestosterone (MENT) (see Fig. 2D) is also of considerable interest.35 This compound is ten times more potent than testosterone and is not converted into dihydrotestosterone, the androgen implicated in prostatic hypertrophy, acne, and male-pattern baldness. To date, MENT has not been used in contraceptive trials, but its potency and tissue selectivity could be advantageous in the long-term safety and side-effect profile of a hormonally derived male contraceptive. MENT (and other new tissue-selective androgens) have the exciting potential of improving general health by decreasing the likelihood of the development of prostatic hypertrophy and prostate cancer in men, in addition to providing safe and effective contraception.
Testosterone with progestins
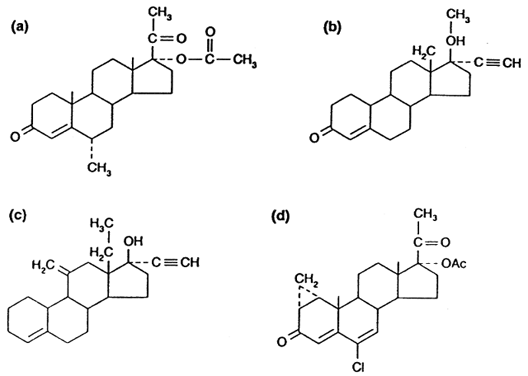
The idea of using a progestin synergistically with testosterone to block sperm production has been extensively tested.36 Combinations of testosterone and depot injections of medroxyprogesterone acetate (DMPA) (Fig. 3A) were shown to induce azoospermia in half of study subjects with some degree of oligozoospermia in most others. The contraceptive efficacy of these combinations, however, was poor, with several couples conceiving while receiving therapy despite simultaneous use of other contraceptives.37
Studies of progestins have focused on newer compounds, such as the potent oral progestin, levonorgestrel (LNG) (see Fig. 3B). A trial of LNG (500 μg orally daily) with TE (100 mg intramuscularly per week) showed the LNG-TE combination was superior to TE alone in terms of azoospermia (67% versus 33%) by 6 months.38 In addition, 94% achieved either severe oligozoospermia or azoospermia in the LNG-TE group compared with 61% of the TE-alone group. Drawbacks to the LNG-TE regimen included greater weight gain and decreases in HDL cholesterol when compared with the TE-alone group. Subsequently, lower doses of LNG have been demonstrated to be as effective at achieving azoospermia with less weight gain and smaller reductions of HDL cholesterol.39 Other progestins, such as desogestrel (see Fig. 3C), have been tested in male contraceptive regimens. When combined with 50- and 100-mg doses of weekly TE, 18 of 23 patients became azoospermic after 24 weeks and all but one suppressed to less than 3 million sperm per milliliter.40 Another study has shown that 100 mg TE weekly with 150 μg desogestrel daily may be more effective than TE/LNG at suppressing sperm counts without causing greater weight gain or larger drops in HDL cholesterol than have been seen with TE-LNG.41
NON-HORMONAL MALE CONTRACEPTION
Adjudin
Adjudin is compound that functions as a contraceptive by disrupting the adhesion of spermatids to Sertoli cells, causing premature spermiation and infertility.41, 42, 43, 44 Administration of two doses of 50 mg/kg of adjudin (one dose per week) induced 100% infertility 5 weeks after treatment in adult rats. Fertility rebounded by week 11.43, The serum testosterone, FSH, and LH levels did not change. Because there was some liver inflammation observed in a 29-day study of adjudin administration, researchers conjugated adjudin to a FSHb mutant specifically targeting it to Sertoli cells, thereby significantly reducing the dose necessary for contraception.44, Unfortunately, the cost of this approach and the possibility of developing anti-FSH autoantibodies is a concern that needs to be addressed as the compound progresses to human studies.
H2-Gamendazole
H2-Gamendazole is a compound that works by impairing the function of the apical ectoplasmic specialization.45 All male rats who received a single oral dose of gamendazole at 6 mg/kg were infertile, but only 57% regained fertility.46 Importantly, there was no significant difference in the circulating LH and testosterone levels compared to the control group at this dose, but FSH did increase, probably due to a reduction in inhibin B release from Sertoli cells. It should be noted that the mating behavior of the treated rats returned to normal and the F1 offspring of the animals who regained fertility did not seem to be affected. In terms of toxicology, three out of five rats died after receiving a dose of 200 mg/kg of H2-gamendazole; however, no observable abnormalities including liver inflammation, necrosis or hemorrhage was detected at dosages lower than 200 mg/kg. As a result, researchers are preparing an IND application to proceed to human testing, probably at doses of 1 mg/kg daily.
Retinoic acid inhibition
It has been known since 1925 that vitamin A (retinol) is required for normal spermatogenesis.47 Nutritional studies in rats and genetic studies in mice demonstrate a requirement for vitamin A and its metabolites at puberty for initiation of spermatogenesis and in adults for maintenance of spermatogenesis.48, 49 In the seminiferous tubules, both germ and Sertoli cells synthesize retinoic acid from retinal via aldehyde dehydrogenases. Retinoic acid binds one of several retinoic acid receptors (RARs), which regulate gene expression. Male RAR knockout animals are sterile due to various problems in spermatogenesis.50, 51, 52, 53 Clearly, blockade of retinoic acid function or synthesis has the potential to inhibit spermatogenesis and is an appealing approach to male non-hormonal contraceptive development.
BMS-189453
BMS-189453 is an orally active retinoic acid receptor antagonist. At daily oral doses of 15, 60, or 240 mg/kg for 1 month, BMS-189453 produced marked testicular degeneration in rats, but also led to increases in leukocyte counts, alkaline phosphatase and alanine aminotransferase levels.54 Significant overt signs of toxicity and deaths occurred at 240 mg/kg, whereas body weight and food consumption decreases occurred at 60 and 240 mg/kg. When BMS-189453 was administered to male rats at daily doses ranging from 12.5 to 100 mg/kg for 1 week, only minimal testicular changes occurred at all doses. This study, however, did not explore the fertility status of the animals nor did it look at reversibility of testicular changes after the study period. A recent nicely done study in mice explored whether a lower dose of BMS-189453 might function as a contraceptive without the toxicity seen at higher doses.55 Two groups of 30 mice each were given BMS-189453 in oral dose of 5 mg/kg for 2 weeks (group 1) and 2.5 mg/kg for 4 weeks (group 2). The study showed that the mice were completely sterile by 4 weeks after a dosing regimen of 5 mg/kg and by the end of treatment with a dose of 2.5 mg/kg for 4 weeks. Infertility persisted for 2 weeks at the highest dose after cessation of treatment and was completely restored by 20 weeks in all but 1 male. In group 2, all males became infertile by the end of the treatment and infertility lasted for 4 weeks after cessation of treatment. By 12 weeks after treatment, fertility was completely restored in all males. The comparison of spermatogenic cell distribution in seminiferous tubules between the study and the control group at the end of the study showed that most of the tubules recovered completely. Hematology and serum chemistry analysis were unaffected by BMS-189453 administration. This compound, or a more specific retinoic acid-alpha antagonists,56, 57hold promise for non-hormonal contraception.
WIN 18,446
Almost 50 years ago, the oral administration of WIN 18,446 was shown to safely, completely and reversibly inhibit spermatogenesis in many species including man.58, 59, 60 Histologic examination of testicular biopsies from men in these studies revealed a complete arrest of spermatogenesis, with an absence of forms beyond spermatogonia.60 Since these compounds had no effect on the endocrine function of the testes, and were not androgenic themselves, it was concluded that the inhibition of spermatogenesis exhibited by WIN 18,446 was not hormonal in nature. At least 60 men were administered WIN 18,446 for up to 1 year with achievement of sperm concentrations below 1 million sperm/ml of ejaculate61—a sperm concentration associated with excellent contraceptive efficacy in trials of male hormonal contraceptives.27 Unfortunately, subjects taking WIN 18,446 experienced a “disulfiram reaction” consisting of nausea, vomiting, palpitations and sweating, when they drank alcohol.59 Because of this, further development of WIN 18,446 was abandoned without an understanding of the mechanism by which it inhibited spermatogenesis.
Our group has recently demonstrated that WIN 18,446 suppresses spermatogenesis by inhibiting testicular retinoic acid biosynthesis.62 In vitro, WIN 18,446 inhibits the function of aldehyde dehydrogenase 1a2, which functions in testicular retinoic acid biosynthesis. Using a rabbit model, we observed that oral administration of WIN 18,446 induced reversible azoospermia. This reduction in spermatogenesis was preceded by a reduction in intratesticular retinoic acid. These findings demonstrate that WIN 18,446 functions as an oral, non-hormonal male contraceptive by inhibiting the testicular biosynthesis of retinoic acid and suggests that inhibition of the testicular retinoic acid biosynthesis is a promising target for male contraceptive development. The development of novel, specific compound that inhibits testicular retinoic acid biosynthesis without interfering with alcohol metabolism is underway, and will hopefully result in compounds that reversibly inhibit spermatogenesis without significant side-effects.
CONCLUSIONS
Additional contraceptives, especially those intended for men, are needed to check population growth and prevent undesired pregnancy. Existing male methods of contraception, including condoms and vasectomy, are effective but have limitations. Research has progressed slowly; however, the hormonal approach to male contraception is effective, reversible, and seems safe. Long-acting injections of testosterone esters (e.g. testosterone undecanoate) may prove effective in combinations with long-acting progestins. Current research is focused on both improving the method and characteristics of androgen administration and finding combinations with progestins that optimize sperm count suppression in all populations while minimizing side-effects. Non-hormonal approaches to male contraception are being pursued, but have not been tested clinically.
REFERENCES
World's population to reach six billion. MMWR, Morb Mortal Wkly Rep 1999;48:859 |
|
Henshaw SK. Unintended pregnancy in the US. Fam Plann Perspect 1998;30:24 |
|
Henshaw SK, Van Vort J. Abortion services in the United States, 1991 and 1992. Fam Plann Perspect 1994;26:100 |
|
Henshaw SK. Induced abortions: A world review. Fam Plann Perspect 1990;22:76 |
|
Maine D, Karkazis K, Bolan N. The bad old days are still here: Abortion mortality in developing countries. J Am Med Womens Assoc 1994;49:137 |
|
Plymate SR. Male hypogonadism. In: Becker KL (ed), Principles and Practice of Endocrinology and Metabolism, p 1056. 2nd ed. Philadelphia: JB Lippincott; 1995 |
|
DeKretser DM. Morphology and physiology of the testis. In: Becker KL (ed), Principles and Practice of Endocrinology and Metabolism, p 1032. 2nd ed. Philadelphia: JB Lippincott; 1995 |
|
Silber SJ, Ord T, Balmaceda J et al. Congenital absence of the vas deferens: The fertilizing capacity of human epididymal sperm. N Engl J Med 1990;323:1788 |
|
Haws JM, Morgan GT, Pollack AE et al. Clinical aspects of vasectomies performed in the United States in 1995. Urology 1998;52:685 |
|
Schmidt SS. Vasectomy by section, luminal fulguration and fascial interposition: Results from 6248 cases. Br J Urol 1995;76:373 |
|
Philp T, Guillebaud J, Budd D. Complications of vasectomy: review of 16,000 patients. Br J Urol 1984;56:745 |
|
Li S-Q, Goltein M, Shu J et al. The no-scapel vasectomy. J Urol 1991;145:341 |
|
Nirapathpongporn A, Huber DJ, Krieger JN. No scalpel vasectomy at the King's birthday vasectomy festival. Lancet 1990;335:894 |
|
Skriver M, Skovsgaard F, Miskowiak J. Conventional or Li vasectomy: A questionnaire study. Br J Urol 1997;79:596 |
|
McMahon AJ, Buckley J, Taylor A et al. Chronic testicular pain following vasectomy. Br J Urol 61992;9:188 |
|
Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol 1997;157:518 |
|
Jequier AM. Vasectomy related infertility: A major and costly medical problem. Hum Reprod 1998;13:1757 |
|
Belker AM, Thomas AJ, Fuch EF et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol 1991;145:505 |
|
Heidenreich A, Bonfig R, Wilbert DM et al. Risk factors for antisperm antibodies in infertile men. Am J Reprod Immunol 1994;31:69 |
|
Peterson HB, Howards SS. Vasectomy and prostate cancer: The evidence to date. Fertil Steril 1998;70:201 |
|
Fu H, Darroch JE, Haas T et al. Contraceptive failure rates: New estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect 1999;31:56 |
|
Turjanmaa K. Allergy to natural rubber latex: A growing problem. Ann Med 1994;26:297 |
|
Levy DA, Khouader S, Leynadier F. Allergy to latex condoms. Allergy 1998;53:1107 |
|
Martin CW, Anderson RA, Cheng L et al. Potential impact of hormonal male contraception: Cross-cultural implications for development of novel preparations. Hum Reprod 2000;15:637 |
|
Glasier AF, Anakwe R, Everington D et al. Would women trust their partners to use a male pill? Hum Reprod 2000;15:646 |
|
Amory JK, Bremner WJ. The use of testosterone as a male contraceptive. Ballieres Clin Endocrinol Metab 1998;12:471 |
|
World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of induced azoospermia and oligozoospermia in normal men. Fertil Steril 1997;65:821 |
|
World Health Organization. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet 1990;336:995 |
|
Bagatell CJ, Heiman JR, Matsumoto AM et al. Metabolic and behavioral effects of high-dose, exogenous testosterone in healthy men. J Clin Endocrinol Metab 1994;79:561 |
|
Meriggiola MC, Marcovina S, Paulsen CA et al. Testosterone enanthate at the dose 200 mg/week decreases HDL cholesterol levels in healthy men. Int J Androl 1995;18:237 |
|
Zhang GY, Gu YQ, Wang XH et al. A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl 1998;19:761 |
|
Behre AM, Abshagen K, Oettel M et al. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: Phase I studies. Eur J Endrocrinol 1999;140:414 |
|
Nieschlag E, Buchter D, VonEckardstein S et al. Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men. Clin Endocrinol (Oxf) 1999;51:757 |
|
Gu Y, Liang X, Wu W et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 2009 94:1901-1915 |
|
Sundaram K, Kumar N, Bardin CW. 7 alpha-methyl-19-nortestosterone: An ideal androgen for replacement therapy. Rec Prog Horm Res 1994;49:373 |
|
Meriggiola MC Bremner WJ. Progestin-androgen combination regimens for male contraception. J Androl 1997;18:240 |
|
Barfield A, Melo J, Coutinho E et al. Pregnancies associated with sperm concentrations below 10 million/ml in clinical studies of a potential male contraceptive method, monthly depot medroxyprogesterone acetate and testosterone esters. Contraception 1979;20:121 |
|
Bebb RA, Anawalt BD, Christensen RB et al. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: A promising male contraceptive approach. J Clin Endocrinol Metab 1996;81:757 |
|
Anawalt BD, Bebb RA, Bremner WJ et al. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl 1999;20:407 |
|
Wu FC, Balasubramanian R, Mulders TIM et al. Oral progestogen combined with testosterone as a potential male contraceptive: Additive effects between desogestrel and testosterone enanthate in suppression of spermatogenesis, pituitary-testicular axis, and lipid metabolism. J Clin Endocrinol Metab 1999;84:112 |
|
Cheng GY, Mruk D. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testes. Spermatogenesis 201; 1:137-146. |
|
Cheng GY, Mruk D. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testes. Spermatogenesis 201; 1:137-146. |
|
Mok K-W, Mruk DD, Lie PPY, Lui W-Y, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction 2011;141:571-580. |
|
Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nature Medicine 2006;12:1323-1328. |
|
Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod. 2008;78:1127-38. |
|
Tash JS, Chakrasali R, Jakkaraj SR et al. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 and EEF1A1, and stimluates II1a transcription in rat Sertoli cells. Biol Reproduction 2008;78:1139-1152. |
|
Wolbach SB, Howe PR. Tissue changes following deprivation of fat soluble A Vitamin. J Exp Med 1925;42:753-777. |
|
Vernet N, Dennefeld C, Rochett-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006;147:96-110. |
|
Koubova J, Menke D, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA 2006;103:2472-2479. |
|
Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal devlopment: identification of potential heterimeric receptors. Biol Reprod 1999;61:1300-1308. |
|
Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA 1993;90:7225-7229. |
|
Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell 1993;73:643-658. |
|
Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Décimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev 1996;10:80-92. |
|
Schulze GE, Clay RJ, Mezza LE, Bregman CL, Buroker RA, Frantz JD. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol Sci 2001;59:297-308. |
|
Chung SS, Wang X, Roberts SS, Griffey SM, Reczek PR, Wolgemuth DJ. Oral administration of a retinoic Acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology. 2011;152:2492-502 |
|
Chung SS, Sung W, Wang X, Wolgemuth DJ. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn 2004;230:754-66. |
|
Chung SS, Wang X, Wolgemuth DJ. Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalties in spermiogenesis. Differentiation 2005;73:188-198. |
|
Coulston F, Beyler AL, Drobeck HP. The biologic actions of a new series of bis(dichloroacetyl) diamines. Toxicol Appl Pharmacol 1960;2:715-721. |
|
Beyler AL, Potts GO, Coulston F, Surrey AR. The selective testicular effects of certain bis-(dichloroacetyl) diamines. Endocrinology 1961;69:819-833. |
|
Heller CG, Flageolle BY, Matson LJ. Histopathology of the human testes as affected by Bis (dichloroacetyl) diamines. Exp Mol Path 1963;2:107-114. |
|
Heller CG, Moore DJ, Paulsen CA. Suppression of spermatogenesis and chronic toxicity in men by a new series of Bis (dichloroacetyl) Diamines. Toxicol Appl Pharmacol 1961;3:1-11. |
|
Amory JK, Muller CH, Shimshoni AJ, Isoherranen N, Paik J, Moreb JS, Amory DW, Evanoff R, Goldstein AS, Griswold MD. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl 2011; 32:111-119. |